Quality Management
What Quality means to Fresenius Kabi Oncology Limited
The commitment to improving a patient’s quality of life is invaluable to Fresenius Kabi Oncology Limited. Therefore, the Company adheres to effective Quality Management procedures in order to ensure high level of safety and efficacy in our products. Our manufacturing operations follow the cGMPs and all other necessary certifications and guidelines to maintain total compliance with Pharmaceutical Regulatory Authorities’ norms and thereby meeting the expectations of our partners and customers.
With the aim to assist the medical professionals in providing the best possible therapy and care for cancer patients globally, we consistently thrive for continuous quality improvement across all areas in our operations.
At Fresenius Kabi Oncology Limited, we try to ensure that every individual accepts to thrive for continuous improvements over our systems and processes as their goal. Also, effective decision-making based on the analysis of data and information is an important aspect of our operations management.
Our commitment to continuously offer products and services with high quality and efficacy is the foundation of our long-lasting and successful partnerships with our customers. Therefore, according to the international ISO-9001 Standards, our quality management system is regularly reviewed during internal quality audits and certified by external auditors.
Good documentation is an
essential part of the QM System and is a prerequisite for compliant operations.
Generally, there are two primary types of documentation:
a) Instructive documents which define and direct all quality impacting activities like e.g.,
Handbooks, SOPs, Guiding Documents, Working Instructions, test plans, sampling
plans, templates and
b) Records or reports that document the execution of those activities (e.g., manufacturing and laboratory testing records, audit reports, validation reports, batch records, sampling records, etc.).
The instructive global QM Documents
are made available to all parties involved in electronic format. Information on
new or revised global QM Documents is actively communicated.
Upon receipt, the recipient
has the responsibility to assess new or revised QM Documents for applicability
to their products, systems, processes and business operations.
If applicability is
identified, the respective QM Documents are implemented effectively into the
local QMS.
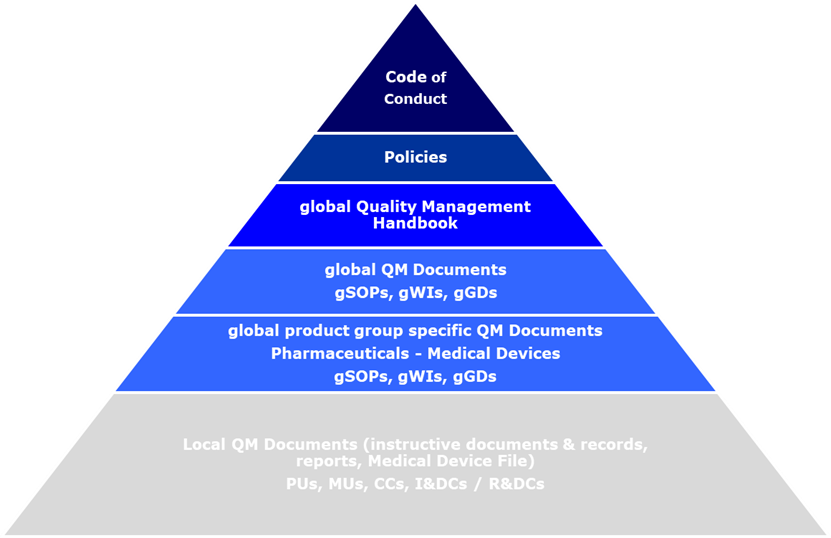
Quality Management Documents Structure
